While you are under general anesthesia, your doctor will make a small cut in your upper thigh, near the groin, to access your femoral vein. They will then insert a tube-like device called a delivery catheter. The implant is attached to the tip of the delivery catheter.
Mitral and tricuspid valve repair

What is transcatheter valve repair?
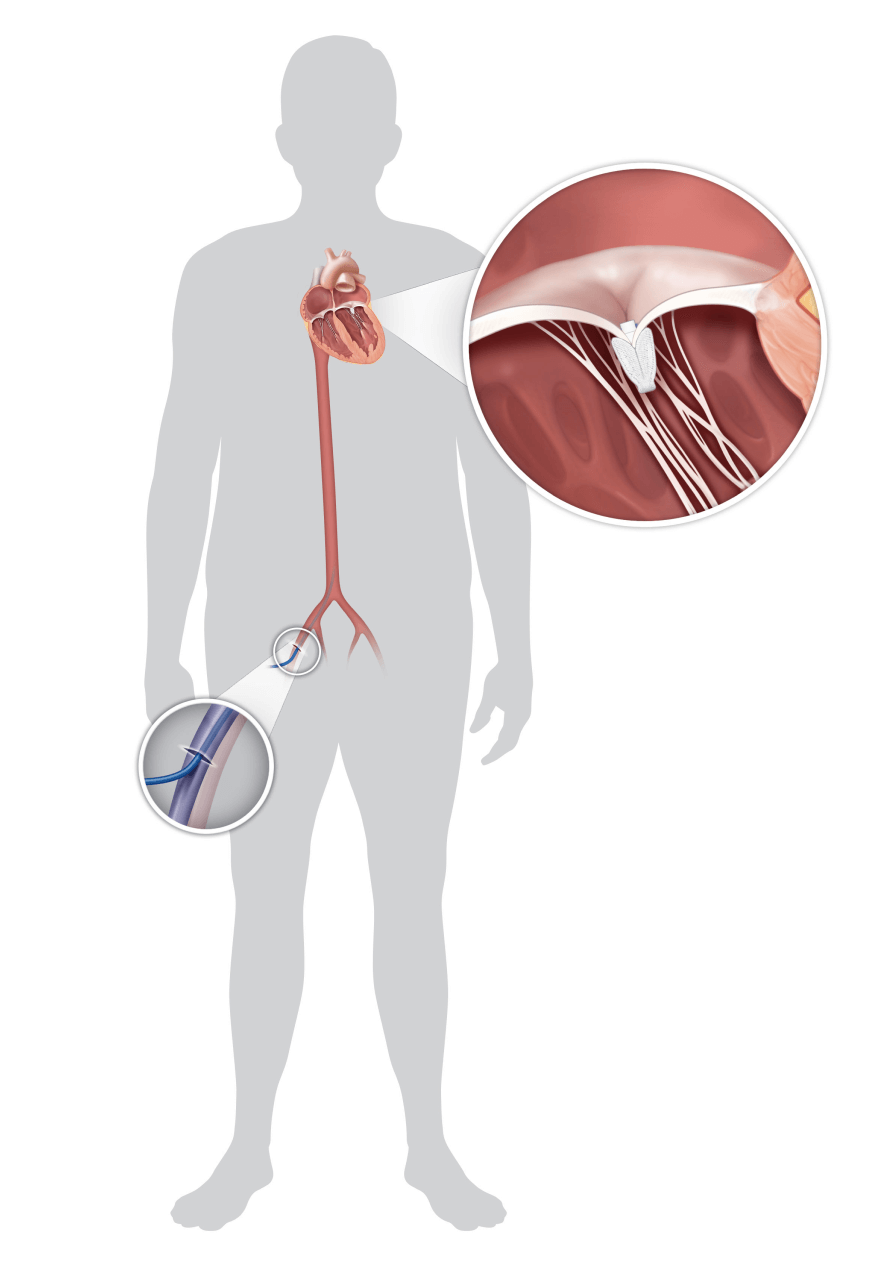
Transcatheter mitral valve repair (TMVr) and transcatheter tricuspid valve repair (TTVr) are catheter-based procedures to repair mitral and tricuspid valves.
Unlike traditional heart surgery, in which the surgeon cuts through the chest wall and then the heart to access the valve, TMVr and TTVr involve implanting a device through a small incision in your upper thigh, near the groin, using a flexible tube called a catheter.

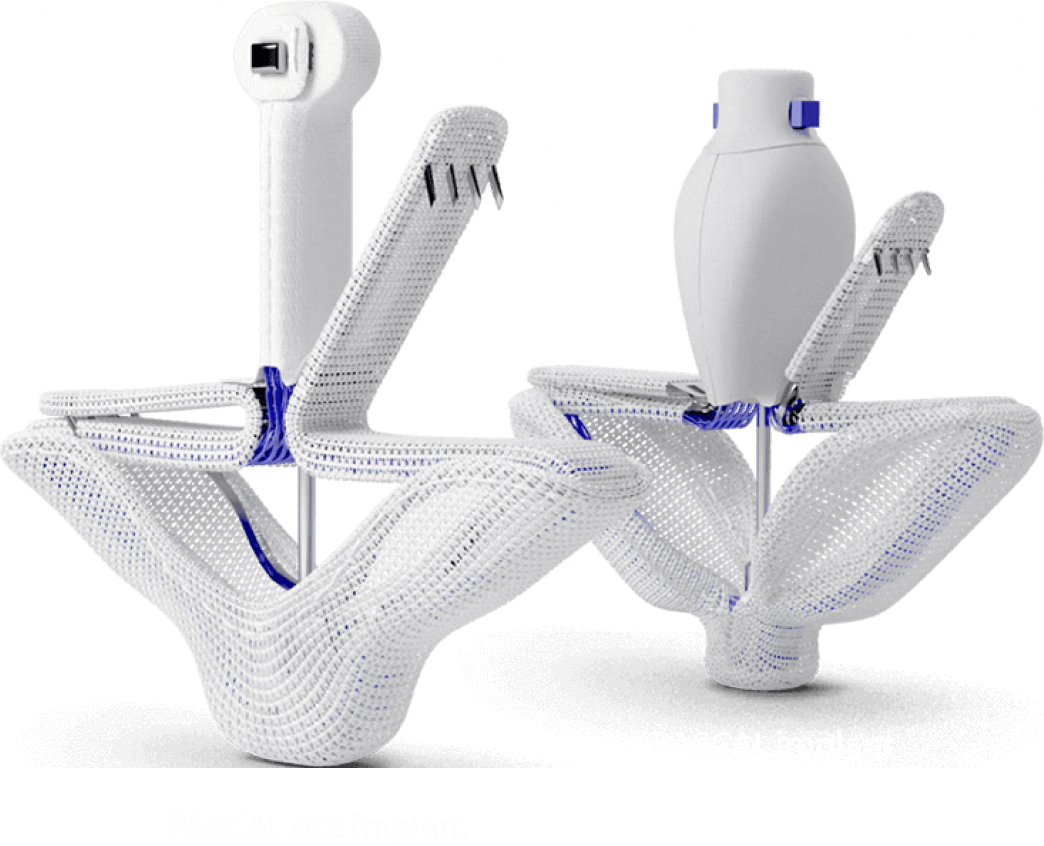
The PASCAL implant

The Edwards PASCAL Precision transcatheter valve repair system is designed to repair the tricuspid or mitral valve and reduce tricuspid or mitral valve regurgitation.1
The PASCAL and PASCAL Ace implants are made of nitinol (nickel and titanium) and have clasps that bring the leaflets of the mitral or tricuspid valve together. The PASCAL Ace is the smaller of the two implants. Your doctor will determine the best implant option for your valve.
What to expect from a transcatheter valve repair procedure
Repairing your mitral valve
The implant will be guided to your mitral valve using imaging equipment.
The implant will be positioned to clasp together your mitral leaflets to reduce the blood leak.
After verifying the final position of the implant, your doctor will release it from the delivery system. The implant will stay in your heart.1
Reduction in annual heart failure hospitalization rate with the PASCAL repair system*2
of patients achieved moderate to no mitral regurgitation with the PASCAL repair system*2
*Results at two-year post-procedure follow-up
Repairing your tricuspid valve
While you are under general anesthesia, your doctor will make a small cut in your upper thigh, near the groin, to access your femoral vein. They will then insert a tube-like device called a delivery catheter. The implant is attached to the tip of the delivery catheter.
The implant will be guided to your tricuspid valve using imaging equipment.
The implant will be positioned to clasp together your tricuspid leaflets to reduce the blood leak.
After verifying the final position of the implant, your doctor will release it from the delivery system. The implant will stay in your heart.1
of patients achieved moderate to no tricuspid regurgitation with the PASCAL repair system*3
Procedural success was achieved in 88% of patients with the PASCAL repair system**3
*Results at one-year post-procedure follow-up
**Procedural success: Implantation of at least one device with post-procedural TR of a moderate or less grade, with no device-related complication, mortality or conversion to surgery

A safe, effective
treatment option

The PASCAL repair system has been studied and shown to be safe and effective at repairing mitral and tricuspid valves in patients with significant regurgitation.1–3
After fixing your mitral or tricuspid valve, you may notice fewer symptoms, improved quality of life and increased ability to exercise.2–4
As with any implanted medical device, there are risks associated with this procedure. Talk to your doctor for a full explanation of the benefits and risks.1
Frequently asked questions
What can I expect from the procedure and recovery?
Learn more about the procedure and what to expect from transcatheter valve repair with the PASCAL Precision system.


Download resources
Get answers to your questions about mitral regurgitation, tricuspid regurgitation and treatment in these downloadable PDFs.

References
- Edwards Lifesciences. Edwards PASCAL Precision Transcatheter Valve Repair System. Instructions for Use. Available at: https://www.accessdata.fda.gov/cdrh_docs/pdf22/P220003D.pdf [Accessed 27 April 2023].
- Szerlip M, et al. 2-Year Outcomes for Transcatheter Repair in Patients With Mitral Regurgitation From the CLASP Study. JACC Cardiovasc Interv. 2021 Jul 26;14(14):1538-1548. Erratum in: JACC Cardiovasc Interv. 2022 Jul 11;15(13):1395.
- Kodali S, et al. 1-Year outcomes of transcatheter tricuspid valve repair. J Am Coll Cardiol. 2023 May 9;81(18):1766-1776. doi: 10.1016/j.jacc.2023.02.049.
- Kitamura M, et al. 12-Month outcomes of transcatheter tricuspid valve repair with the PASCAL system for severe tricuspid regurgitation. Catheter Cardiovasc Interv. 2021 May 1;97(6):1281-1289.
Medical device for professional use.
For a listing of indications, contraindications, precautions, warnings, and potential adverse events, please refer to the Instructions for Use (consult eifu.edwards.com where applicable).
Edwards, Edwards Lifesciences, the stylized E logo, CLASP, Edwards PASCAL, Edwards PASCAL Precision, PASCAL, and PASCAL Precision are trademarks or service marks of Edwards Lifesciences Corporation. All other trademarks are the property of their respective owners.
© 2023 Edwards Lifesciences Corporation. All rights reserved. PP--EU-5802 v1.0
Edwards Lifesciences • Route de l’Etraz 70, 1260 Nyon, Switzerland • edwards.com
